Since late 2019, the coronavirus has grown into a pandemic and forced half of the globe to dramatically self-quarantine. Even if young and healthy people have been shown to have lower chances of developing serious complications from COVID-19, no one knows for sure how their body would react if they became infected. For people over 65 or with certain pre-existing conditions, the impact can be greater. Moreover, patients undergoing treatment for or who are in remission from certain types of cancers are at risk for complications due to COVID-19. So how is the coronavirus pandemic impacting cancer patients and their treatment so far?
1. Underlying medical conditions increase the risk of severe illness from COVID-19
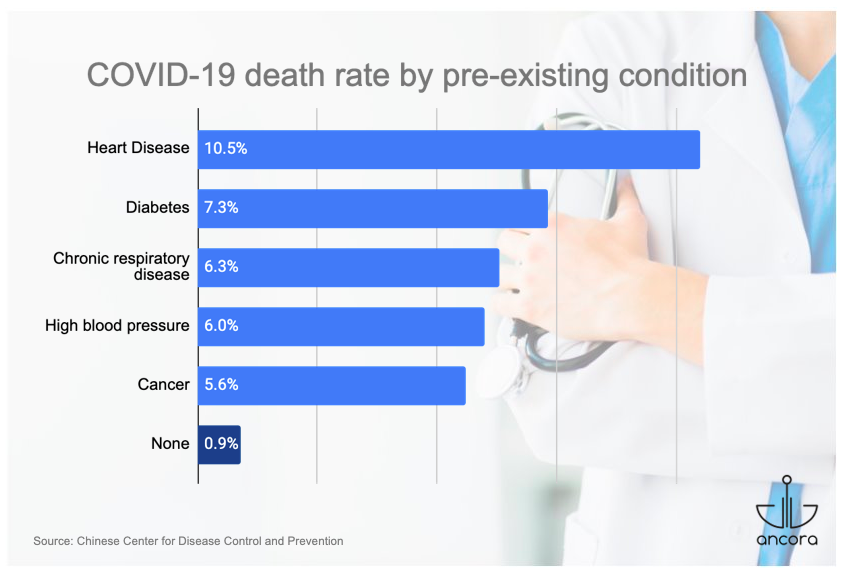
Both cancer treatment and cancer itself may cause cancer patients to be immunocompromised, meaning they have a weakened immune system that is less able to fight infections. For these reasons, cancer patients are at higher risk of developing complications from COVID-19. A report from China showed that the mortality rate for COVID-19 patients with an underlying cancer was higher (5.6%) than for patients without any preexisting conditions (0.9%) but lower than for patients suffering from diabetes and heart disease.[1]
Patients who are currently undergoing chemotherapy, have low levels of white blood cells and/or are taking immune-suppressive agents have a weakened immune system and therefore are more likely to develop health problems if they contract the virus.[2][3]
2. Cancer treatment has been disrupted by the COVID-19 pandemic
As COVID-19 cases skyrocket, hospital facilities around the world are being overwhelmed by waves of patients. In many European countries, and now also in the U.S., shortages of staff, beds, and other medical resources are unfortunately becoming a common problem, which also poses challenges to oncology patients. Healthcare providers are facing numerous ethical dilemmas and are having to postpone or put on hold many cancer surgeries, screenings, therapies and other medical procedures that are not considered urgent or life-threatening. These tough decisions are made on a case-by-case basis in order to reduce the risk of infection of cancer patients by the coronavirus and to ensure healthcare providers have the resources they need to treat patients seriously affected by COVID-19.
3. The coronavirus pandemic has resulted in delayed cancer clinical trials
While more than 100 new clinical trials are now recruiting for coronavirus research, many ongoing clinical trials for other diseases have been suspended and the start of new trials have been delayed.[4] This is also true for cancer clinical trials many of which have been disrupted, leaving some cancer patients in a difficult situation.
As the healthcare system is overwhelmed by the COVID-19 pandemic, there are uncertainties about the pharmaceutical supply chain, medical resources are being reallocated and patients are facing travel restrictions. All of these issues have implications for cancer clinical trials in progress.
According to a survey conducted by Continuum Clinical last week, clinical trial sites have faced challenges recruiting patients for new trials and are struggling to keep the ones already enrolled compliant with study protocols. Clinical trial professionals also believe that patients are less likely to enroll in new trials at the moment and that patients already enrolled are less likely to continue participation.[5][6]The consequences of this will be that data from pivotal clinical trials will be missing, which will delay drug filings, meaning new drugs may take longer to reach the market.
4. The coronavirus pandemic has hindered cancer research progress
Spring is usually the time of the year when large cancer research meetings, such as the ASCO (American Society of Clinical Oncology) and the AACR (American Association for Cancer Research) take place. These events are where thousands of experts in the field announce and discuss exciting new research, novel treatments, potential collaboration and set goals to advance the field. However, as travel restrictions and social distancing measures were put into place, important events have been canceled or postponed. The cancellation of these events is likely to have a financial impact on these societies and organizations which could affect their funding and their future activities.[7][8]
The pandemic has also forced medical research laboratories and university campuses to shut down in order to protect their workers and keep them at home. Unfortunately, without access to laboratories, scientists cannot progress their research and ongoing projects are jeopardized.[9][10]
5. The pandemic has interfered with the drug supply chain
As you can see Both Europe and the U.S rely heavily on China and India for the supply of medications or their active pharmaceutical components. China’s manufacturing capacities have already been affected by the coronavirus pandemic and India has already decided to limit the export of 26 drugs and drug ingredients in order to treat its own population, meaning the threat of facing a shortage of medicines worldwide, including cancer drugs, is growing.[11][12][13]
The U.S. Food and Drug Administration (FDA) has already added one drug to the drug shortage list and it has identified 20 drugs that might be vulnerable because China is the only source producing their key ingredients.[14] Moreover, many drug producers also operate in northern Italy, which is at the center of the COVID-19 epidemic in Europe, and has seen a slowdown in all its operations due to the restrictions imposed upon the lockdown.
References
- The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) | China CCDC, February 17, 2020 | Accessed: 2020-03-30
- Common Questions About the New Coronavirus Outbreak | American Cancer Society | Accessed: 2020-03-30
- First US clinical trial of Covid-19 vaccine candidate begins | Clinical Trials Arena | Accessed: 2020-04-16
- A guide to clinical trials disrupted by the coronavirus pandemic| BioPharma Dive | Accessed: 2020-03-30
- COVID-19 Live Updates | Continuum Clinical | Accessed: 2020-03-31
- Survey Indicates COVID-19 to Negatively Impact Clinical Trial Enrollment and Retention | Business Wire | Accessed: 2020-03-31
- Survey Indicates COVID-19 to Negatively Impact Clinical Trial Enrollment and Retention | Business Wire | Accessed: 2020-03-31
- How Will COVID-19 Impact Cancer Research? | Everyday Health | Accessed: 2020-03-31
- Coronavirus pandemic forces some research labs to shut down | STAT | Accessed: 2020-03-31
- Updated: Labs go quiet as researchers brace for long-term coronavirus disruptions | Science | AAAS | Accessed: 2020-03-31
- Coronavirus is Creating a Crisis for the U.S. Drug Supply | Time | Accessed: 2020-03-30
- Europe could face more drug shortages as coronavirus squeezes supplies | Reuters | Accessed: 2020-03-30
- Will Coronavirus Stop You From Getting Your Medication? An Expert Answers Your Questions | The National Interest | Accessed: 2020-03-30
- First Drug Shortage Caused by Coronavirus, F.D.A. Says | The New York Times | Accessed: 2020-03-30
