Choice of treatment option for metastatic recurrent cervical cancer is sparse. Development of new treatments is urgently needed. New therapies are currently under investigation in clinical trials and are showing some encouraging early results.
There is a high unmet medical need in second-line cervical cancer treatment
With an estimated 570,000 new cases in 2018[1] (6.6% of all cancers among women), cervical cancer is the fourth most commonly diagnosed type of cancer and the leading cause of death from cancer in women. It is a type of cancer that occurs in the cells of the cervix – the lower part of the uterus that connects to the vagina. It can affect the deeper tissues of the cervix or in some cases, it can metastasize to other parts of the body, such as the vagina, rectum, bladder, lungs, and liver. Cervical cancer is nearly always caused by infection by human papillomavirus (HPV), which is now preventable with a vaccine. It is most often diagnosed in women aged 35 to 44 years old, while about 20% of all cases are diagnosed in women older than 65[2].
Cervical cancer is usually treated with surgery and radiotherapy. Other treatments include chemotherapies and biological therapies. However, when the cancer has metastasized (spread) to other parts of the body and is not responding to treatment (refractory cancer) or comes back after a previous treatment (recurrent cancer), the choice of treatment options becomes sparser and patient outcomes become poorer.
A new drug is showing some encouraging results
A ray of hope for these patients could come from a new therapy currently under investigation in clinical trials that has shown some encouraging early results. Indeed, balstilimab, a drug developed by the immuno-oncology company Agenus, has been recently granted Fast Track Designation by the U.S. Food and Drug Administration (FDA) for both balstilimab as a monotherapy and in combination with zalifrelimab for the treatment in second line of advanced cervical cancer[3]. This designation means the FDA will facilitate the development and review of the new therapies and could potentially lead to accelerated approval.
Balstilimab and zalifrelimab are investigational anticancer therapies, called immune checkpoint inhibitors.
- Balstilimab works by blocking the activity of the protein PD-1.
- Zalifrelimab works by blocking the activity of the protein CTLA-4.
Immune checkpoint inhibitors are a type of immunotherapy. The immune system is usually able to recognize tumor cells and other intruders like viruses and bacteria and attacks them. However, tumor cells are able to hide from the immune system by stimulating the immune system’s brakes or so-called immune checkpoints. Immune checkpoint inhibitors drugs are designed to release the brakes turned off by tumor cells so that the immune system can recognize and kill tumor cells. In 2018, the Nobel Prize in Medicine was awarded for the establishment of PD-1 as a cancer immunotherapy target.
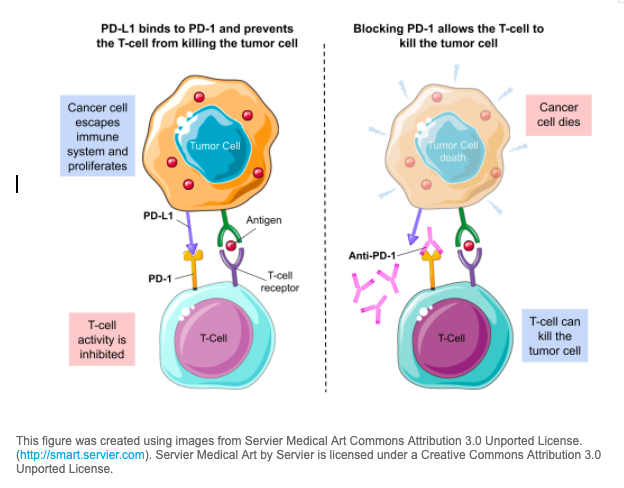
This figure was created using images from Servier Medical Art Commons Attribution 3.0 Unported License. (http://smart.servier.com). Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License.
Balstilimab may help shrink tumors
Balstilimab plus Zalifrelimab combination therapy was investigated in a population of patients who were enrolled irrespective of any biomarkers and with refractory cervical cancer who failed prior platinum chemotherapy with or without bevacizumab. The interim data[4] showed that, out of 34 evaluable patients:
- 4 patients showed complete response rates (CRs)
- 5 patients showed partial response rates (PRs)
- 8 patients had stable diseases (SDs)
⇒ Combined together these data result in an objective response rates (ORR) of 26.5%
Balstilimab monotherapy also showed positive early results, as out of 44 patients:
- 1 patient who showed a complete response (CR)
- 5 patients who showed partial response rates (PRs)
⇒ Combined together these data result in an objective response rates (ORR) of 14.3%
Some important definitions:
- ORR: Objective response rate is the proportion of patients in a clinical trial whose tumor is destroyed or significantly reduced by a drug. ORR is usually the sum of complete responses (CRs) – patients who don’t show any detectable evidence of a tumor over a specific period of time – and partial responses (PRs) – patients who show a decrease in their tumor size over a specific period of time. An improved ORR is a tangible indication that a drug is working.
- SD: Stable disease is defined when the tumor is neither decreasing or increasing in extent or severity.
If you are interested in this treatment, there are currently several clinical trials recruiting patients to test balstilimab (also known as AGEN2034) and or Zalifrelimab (also known as AGEN1884) in various medical settings in the U.S. (E.g. https://www.ancora.ai/details/NCT03894215).
Have a look at Ancora.ai to find clinical trials matching your needs.
References
- Cervical Cancer | WHO
Accessed: 2020-06-04 - Cervical Cancer: Statistics | Cancer.Net
Accessed: 2020-06-04 - FDA Grants Fast Track Designation for Balstilimab to Treat Advanced Cervical Cancer | Cancer Network
Accessed: 2020-06-04 - Agenus Receives Fast Track Designation for Balstilimab in Advanced Cervical Cancer – Apr 7, 2020 | Agenus
Accessed: 2020-06-04
